Bellen continues discharging
its moral and legal obligations to IP, EHS and Quality in a professional
manner. As the "water-carrier" for new drug research and development,
we regard compliance as the lifeline and the foundation of sustainable
development.
Bellen demonstrates zero
tolerance with respect to protection of Intellectual Property and is committed
to global sustainability of environmental protection and safe working practices.
The dynamic leadership shown by senior management team and demonstrable
personal accountability of employees throughout the organization, facilitates
safe delivery of products and services of the highest quality, thereby helping
to ensure long term win-win with our customers.

We know that intellectual property is the most essential property of our customers. Respecting confidentiality, protection of intellectual property is the obligation and responsibility of everyone in Bellen, and it is also one of our priorities. We continue to improve the intellectual property protection management system, use advanced technology, and develop a set of strict and effective protection of intellectual property and information security processes to ensure customers' IP and any confidential information is effectively protected.
Upon collaboration, we will sign a confidentiality agreement and master agreement with the customer. Under the confidentiality agreement, both parties shall carry out technical data sharing and technical discussion. The confidentiality obligation starts from customer's inquiry, and runs through the entire project implementation process. Transfer, control, and store of confidential information are controlled by the corresponding system and process. Our IP team organizes training, audits and daily inspections on a regular or irregular basis to ensure that systems and processes are effectively implemented.
We have introduced the most advanced computer and network behavior management software to effectively restrict and monitor all activities online.
We continue to invest in system design and independently developed a project management system, through which users share data including confidential information. By leveraging the system, offline communication and information transfer are reduced hence data security confidentiality are enhanced. The system can strictly control the access, record and trace back the history of operations of all users as needed.
We consistently regards environment, health, and safety as fundamental components for healthy business development. It is also our basic social responsibility and the driving force for sustainable growth of the company. Our EHS policy clearly outlines the organization's aspirations and provides relevant guidance for our employees. We continually invest in human resources, technology, and capital to establish a comprehensive EHS management system that aligns with international standards and is applicable to our current status. Our EHS management system complies with industry standards and management requirements of multinational companies (MNCs), and we have obtained certification for ISO 14001 Environmental Management System and OHSAS 18001 Occupational Health and Safety Assessment Series.
Regarding production safety, we established a rigorous safety evaluation process based on RC1, ARC, RADEX, DSC, SEDEX, and other professional safety testing and evaluation equipment. Moreover, we've implemented a complete safety production responsibility system for all employees, conducting annual safety education and training, along with regular and special inspections for safety checks and hidden danger identification and rectification. Regarding environmental protection, we actively develop green chemistry and clean production technologies, integrating these technologies and concepts into various stages such as new product development, process optimization, and technological improvements. We focus on utilizing chemistry principles and technologies to reduce pollution at the source.
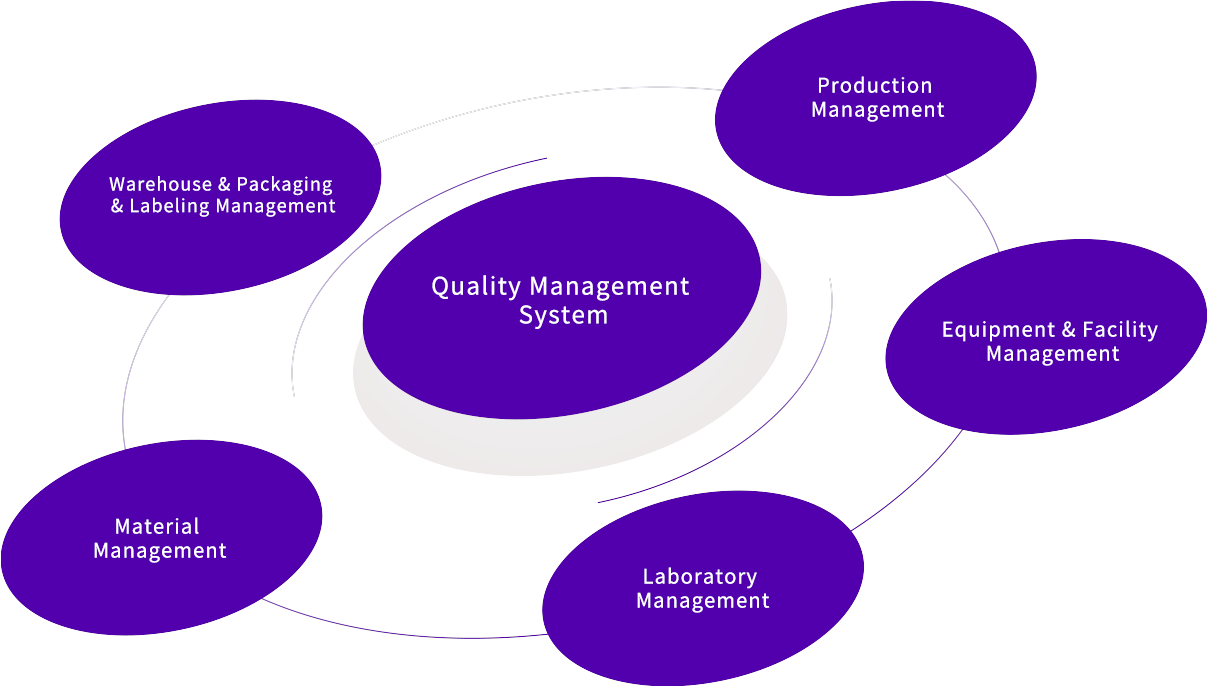
The quality system of Bellen is designed to meet the most stringent global regulatory standards, ensuring that we consistently deliver products and services that meet customer expectations. We recognize the importance of quality management standards as a crucial criterion for selection by global new drug makers when choosing core suppliers. We have established a quality management system composed of six subsystems: quality system as the core, production system, equipment and facility system, material system, packaging and labeling system, and laboratory system. The system adheres to the relevant specifications for drug production quality management, ISO9001 for quality management, and other requirements in our main customer's countries or regions.
Considering the varying requirements for quality management standards in different stages of the chemistry CRO business and chemistry CDMO business, within the framework of a unified quality management system, the company has established a graded quality management system based on stages of the product and customer requirements. This system balances cost and efficiency, implementing a cGMP quality management system in GMP workshops and a moderately cGMP management system in non-GMP workshops. Within the moderate cGMP management system for non-GMP workshops, elements of the cGMP quality management system, such as good record management practices, change management, and deviation management, are retained to maintain high-quality standards. We have successfully undergone over a hundred quality audits from global clients, reflecting the recognition of our company's quality management system by key players in the pharmaceutical industry.